Survive thanks to Research
Clinical Trials Lead Manager: Mag. Michaela Schachner
The medical study activities of the CCCIT are established in the international top league of clinical cancer research. Thus, we are able to offer adjusted therapies to our patients, depending on their individual disease situation.
Our patients have the earliest possible access to the latest cancer therapies which might only receive final approval and standard therapy status in a few years.
There is an ongoing focus on “Phase 1“ studies. Patients, for whom all existing standard therapies have been exploited, will be offered further, intensely monitored therapy options within “Phase 1“ studies.
The participation to these clinical trials is based solely on a voluntary decision and requires upfront an extensive consent discussion. The patient will be given reasonable time to consider their possible participation in the clinical study and to discuss any open questions with the treating physician.
Only after the required consent form has been signed by the patient and submitted, study-related examinations and subsequently the according therapy will begin.
Our highest priority is our patients, their safety and well-being, values anchored as such also in the Mission Statement of the Universitätsklinik für Innere Medizin III.
International ranking of the SCRI-CCCIT
| Tumorart | III. Medizin SCRI-CCCIT | John Hopkins | Cleveland | Standford |
|---|---|---|---|---|
| Ranking USA | – | 4 | 8 | 9 |
| Brustkrebs | 26 | 32 | 30 | 30 |
| NHL | 20 | 18 | 22 | 17 |
| Dickdarmkrebs | 10 | 6 | 15 | 24 |
| Visiten pro Patient | 39.737 | 510.841 | 4.310.000 | 92.875 |
International ranking of the SCRI-CCCIT
| Tumorart | III. Medizin SCRI-CCCIT | John Hopkins |
|---|---|---|
| Ranking USA | – | 4 |
| Brustkrebs | 26 | 32 |
| NHL | 20 | 18 |
| Dickdarmkrebs | 10 | 6 |
| Visiten pro Patient | 39.737 | 510.841 |
| Tumorart | Cleveland | Standford |
|---|---|---|
| Ranking USA | 8 | 9 |
| Brustkrebs | 30 | 30 |
| NHL | 22 | 17 |
| Dickdarmkrebs | 15 | 24 |
| Visiten pro Patient | 4.310.000 | 92.875 |
TumorartIII. Medizin SCRI
Ranking USA–
Brustkrebs 26
NHL20
Dickdarmkrebs10
Visiten pro Patient39.737
TumorartJohn Hopkins
Ranking USA4
Brustkrebs 32
NHL18
Dickdarmkrebs6
Visiten pro Patient510.841
TumorartCleveland
Ranking USA8
Brustkrebs 30
NHL22
Dickdarmkrebs15
Visiten pro Patient4.310.000
TumorartStandford
Ranking USA9
Brustkrebs 30
NHL17
Dickdarmkrebs24
Visiten pro Patient92.875
From the consent discussion until the start of the therapy

The detailed consent discussion takes place with the doctor and the consent form is filled out by the patient

Distribution of study drug after fulfilling all inclusion and exclusion criteria of the study

Examinations are conducted and samples are collected and processed, in compliance with the study guidelines

Start of the therapy

Worldwide samples are collected by the reference laboratory

Manual data entry of all collected patient information into an online database

The detailed consent discussion takes place with the doctor and the consent form is filled out by the patient

Examinations are conducted and samples are collected and processed, in compliance with the study guidelines

Worldwide samples are collected by the reference laboratory

Distribution of study drug after fulfilling all inclusion and exclusion criteria of the study

Start of the therapy

Manual data entry of all collected patient information into an online database
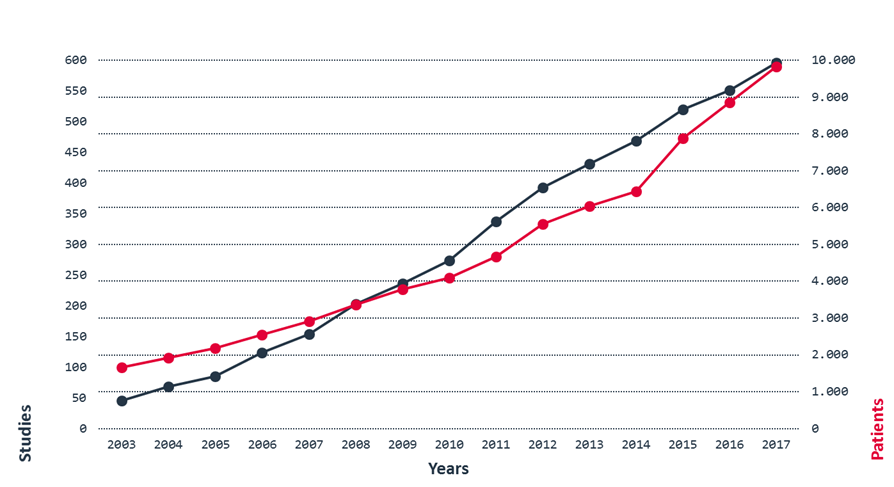
Clinical trials and patients recruited within our studies
Our Current clinical trials
Breast cancer
Colorectal cancer
Gastrointestinal tumor
Hepatocellular carcinoma
Renal cell carcinoma
Lung carcinoma
HNO tumor
Esophageal tumor
Brain tumours/ Glioblastoma
Neuroendocrine tumor
Prostate cancer
Thyroid carcinoma
Ovarian cancer
Melanoma
Soft Tissue Sarcomas
Immune Thrombocytopenia (ITP)
Myelodysplastic syndrome
Chronic myelo-monocytic leukemia (CMML)
Pancreas carcinoma
Plasmacytoma / Multiple myeloma
Mastocytosis
Rheumatoid arthritis
HIV
Diffuse large B Cell Lymphoma
Follicular lymphoma
Marginal-zone lymphoma
Peripheral T-cell lymphoma
Chronic lymphocytic leukaemia (CLL)
Mantle cell lymphoma
Hodgkin disease
Acute myeloid leukaemia (AML)
Acute lymphoblastic leukemia (ALL)
Essential thrombocythaemia (ET)
Polycythemia vera (PV)
Chronic myelogenous leukemia (CML)
Breast cancer
Colorectal cancer
Gastrointestinal tumor
Hepatocellular carcinoma
Renal cell carcinoma
Lung carcinoma
HNO tumor
Esophageal tumor
Brain tumours/ Glioblastoma
Neuroendocrine tumor
Prostate cancer
Thyroid carcinoma
Ovarian cancer
Melanoma
Soft Tissue Sarcomas
Immune Thrombocytopenia (ITP)
Myelodysplastic syndrome
Chronic myelo-monocytic leukemia (CMML)
Pancreas carcinoma
Plasmacytoma / Multiple myeloma
Mastocytosis
Rheumatoid arthritis
HIV
Diffuse large B Cell Lymphoma
Follicular lymphoma
Marginal-zone lymphoma
Peripheral T-cell lymphoma
Chronic lymphocytic leukaemia (CLL)
Mantle cell lymphoma
Hodgkin disease
Acute myeloid leukaemia (AML)
Acute lymphoblastic leukemia (ALL)
Essential thrombocythaemia (ET)
Polycythemia vera (PV)
Chronic myelogenous leukemia (CML)
Recruited patients & number of trials
| solid tumors | N Studies |
|---|---|
| Breast Cancer | 126 |
| Colorectal Cancer | 51 |
| HNO | 17 |
| Gastric Cancer | 18 |
| NSCLC | 27 |
| Pancreas Carcinoma | 15 |
| HCC | 2 |
| RCC | 11 |
| Braintumor | 6 |
| Prostate Cancer | 8 |
| Melanoma | 9 |
| Esophageal Carcinoma | 3 |
| Sarcoma | 2 |
| Thyroid cancer | 3 |
| Neuroendocrine Tumor | 3 |
| Ovarian Cancer | 4 |
| GIST | 4 |
| Other indications | 28 |
| Total | 337 |
| hematological malignancies | N Studies |
|---|---|
| NHL | 78 |
| MDS | 13 |
| MMyelom | 39 |
| AML | 25 |
| CML | 15 |
| HD | 14 |
| Myelofibrosis | 4 |
| ALL | 8 |
| ITP | 3 |
| PV | 3 |
| CMML | 1 |
| ET | 1 |
| Mastocytosis | 1 |
| Other Indications | 22 |
| Total | 337 |
| Rheumatology | N Studies |
|---|---|
| Total | 14 |
| Infectiology | N Studies |
|---|---|
| Total | 18 |
Grand Total 596
solid tumors: N Studies
Breast Cancer: 126
Colorectal Cancer: 51
HNO: 17
Gastric Cancer: 18
NSCLC: 27
Pancreas Carcinoma: 15
HCC: 2
RCC: 11
Braintumor: 6
Prostate Cancer: 8
Melanoma: 9
Esophageal Carcinoma: 3
Sarcoma: 2
Thyroid cancer: 3
Neuroendocrine Tumor: 3
Ovarian Cancer: 4
GIST: 4
Other indications: 28
Total: 337
hematologicalN Studies
malignancies:
NHL: 78
MDS: 13
MMyelom: 39
AML: 25
CML: 15
HD: 14
Myelofibrosis: 4
ALL: 8
ITP: 3
PV: 3
CMML: 1
ET: 1
Mastocytosis: 1
Other Indications: 22
Total: 337
Rheumatology N Studies
Total: 14
Infectiology: N Studies
Total: 18
Grand Total 596
Do you have a request or any questions?
Feel free to contact us – we will get back back to you as soon as possible.